Topic 1:
ncRNAs and their functions
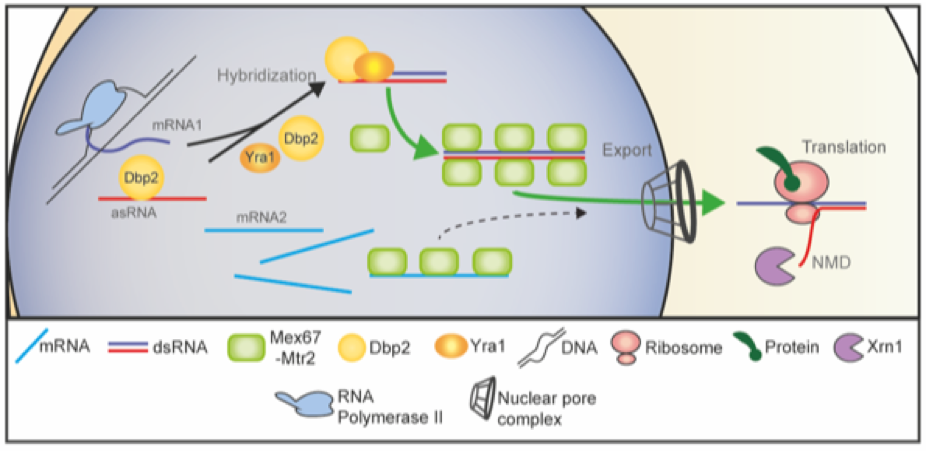
ncRNAs are present in high amounts in all cells. However, the knowledge about their functioins lack far behind the role of e.g. mRNAs. They are seperated in short (below 200 bases) and long (Above 200 bases) ncRNAs. In our research we concentrate on long ncRNA (lncRNAs). We discovered for example that lncRNAs that are antisense to mRNAs boost gene expression by annealing with their counterparts through the helicase Dbp2 and its helicase Yra1 for preferred nuclear export and translation. in this way, the expression of specific mRNAs is boosted. This is of particular interest upon changing environment.
We are also studying other functions of lncRNAs, including functions in translation and we analyze the function of long intergenic ncRNAs.
Telomerase
The telomer ends of the genome have to be elongated because they are shortened after each round of replication. This is accomplished by the telomerase, which is composed of several proteins and the RNA TLC1. We found that TLC1 undergoes a nucleo-cytoplasmic maturation cycle which involves the export factors Mex67 and Xpo1 and the nuclear re-import factors Mtr10 and Cse1.
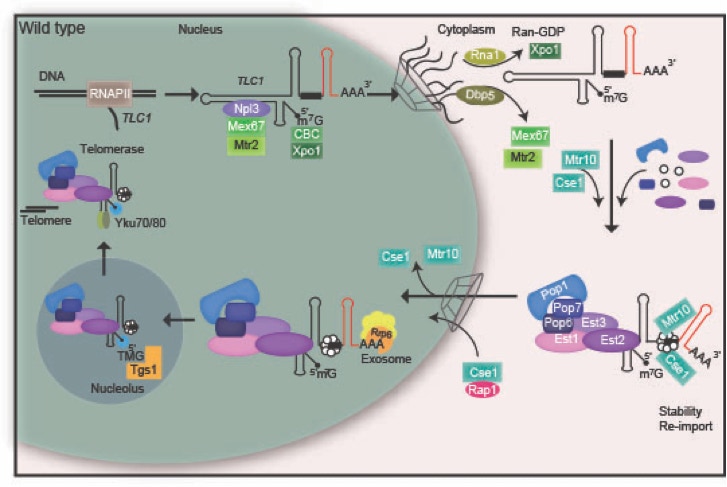
Model for the compartmental stepwise maturation of TLC1. The longer precursor of TLC1 is transcribed in the nucleus and immediately exported into the cytoplasm upon binding of the export receptor Mex67-Mtr2 and the karyopherin Xpo1/Crm1, the latter of which interacts with CBC bound m7G-caps. Upon export, Mex67 and Xpo1/Crm1 are displaced and the Sm-ring, the Est and the Pop proteins assemble on the immature TLC1 in the cytoplasm. Subsequently, this RNP is re-imported back into the nucleus via Mtr10 and Cse1, both of which contact TLC1 via its Sm-ring. Thus, nuclear re-import can only occur after RNP formation including the Sm-ring and therefore resembles an important quality control checkpoint. In the nucleus, the import receptors dissociate and TLC1 is trimmed up to the Sm-ring by the nuclear exosome. Finally TMG-capping occurs in the nucleolus assisted by Smb1, which interacts with Tgs1. This step terminates shuttling, because export receptors cannot be loaded anymore. The matured holoenzyme subsequently acts in telomere maintenance.
Topic 2:
mRNA quality control
One of the main features of eukaryotic cells is the compartmentation between nucleus and cytoplasm. In this way the nuclear intron containing pre-mRNAs are not translated. Quality control mechanisms ensure that pre-mRNAs remain in the nucleus until splicing and processing is completed. However, in case an intron containing mRNA reaches the cytoplasm, this could be harmful for cells and in higher eukaryotes result in diseases such as cancer and neurodegenerative diseases.
Nuclear mRNA quality control
This project focuses on mRNA maturation the identification and characterization of quality control mechanisms in the eukaryotic model organism Saccharomyces cerevisiae. Quality control occurs in the nucleus and and in the cytoplasm. In the nucleus we discovered a group of guarding proteins that are loaded co-transcriptionally on the pre-mRNA. Each maturation step is controlled and after successful 5’-capping, intron splicing and 3’-polyadenylation the guards Npl3, Gbp2, Hrb1 and Nab2 recruit the export receptor heterodimer Mex67-Mtr2 (human TAP-p15) to the matured mRNA for nuclear export. In case processing is not completed successfully, Mex67 is not bound but the guards recruit the nuclear degradation machinery instead to eliminate the faulty transcript.
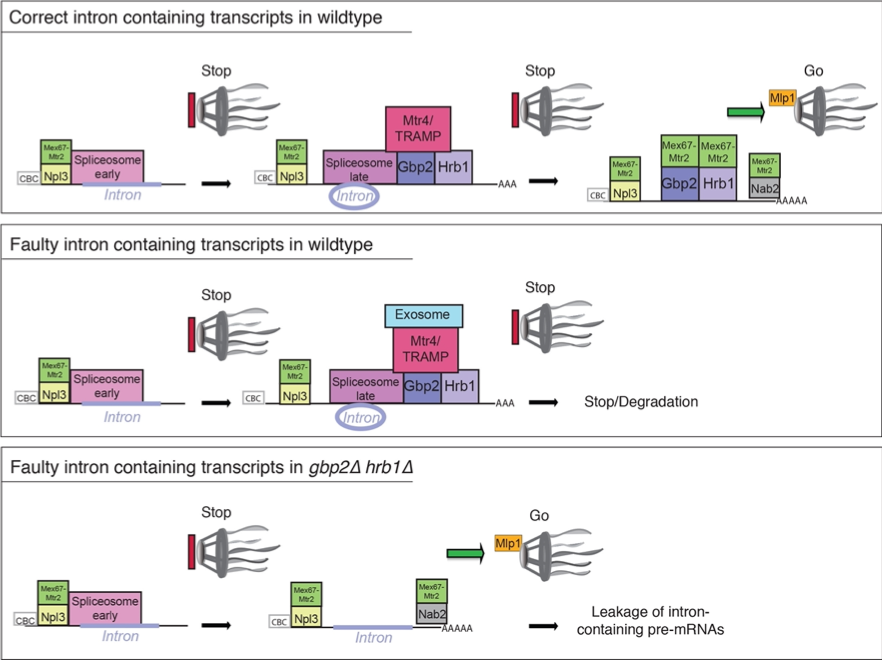
Nuclear guard protein mediated quality control of splicing. Npl3 is recruited to the pre-mRNA early via RNA polymerase II (not shown),where it interacts with the mRNA cap-binding complex (CBC) and the early spliceosome. Npl3 associates with the heterodimer Mex67-Mtr2 to support export, but association of the spliceosome retains the mRNP. Gbp2 and Hrb1 bind at late steps of splicing to the spliceosome and recruit the TRAMP complex to the pre-mRNA. Faulty and unsplicible mRNAs are marked for degradation at the nuclear exosome (middle). On correct mRNPs, Gbp2 and Hrb1 recruit Mex67 to signal the export competence (top). In the absence of Gbp2 and Hrb1 (bottom) faulty pre-mRNAs exit the nucleus, because the surveillance system that monitors the completion of splicing is missing. Nab2 is involved in the 30-end processing and recruits Mex67 upon completion. Proper coverage of the Npl3-, Gbp2, Hrb1- and Nab2-bound mature mRNA with Mex67 is monitored by Mlp1 at the NPC.
Cytoplasmic mRNA quality control
While nuclear mRNA quality control focusses on rather structural features of the mRNA, the cytoplasmic quality control checks the open reading frame (ORFs). This involves the ribosome as the decoding machinery and transcripts with defective ORFs are eliminated in processes called NoGo- (NGD) NoStop- (NSD) and NonsenseMediated-decay (NMD). We could recently show that two of the guard proteins, Gbp2 and Hrb1 are involved in NMD and thus continue their surveillance function in the cytoplasm.
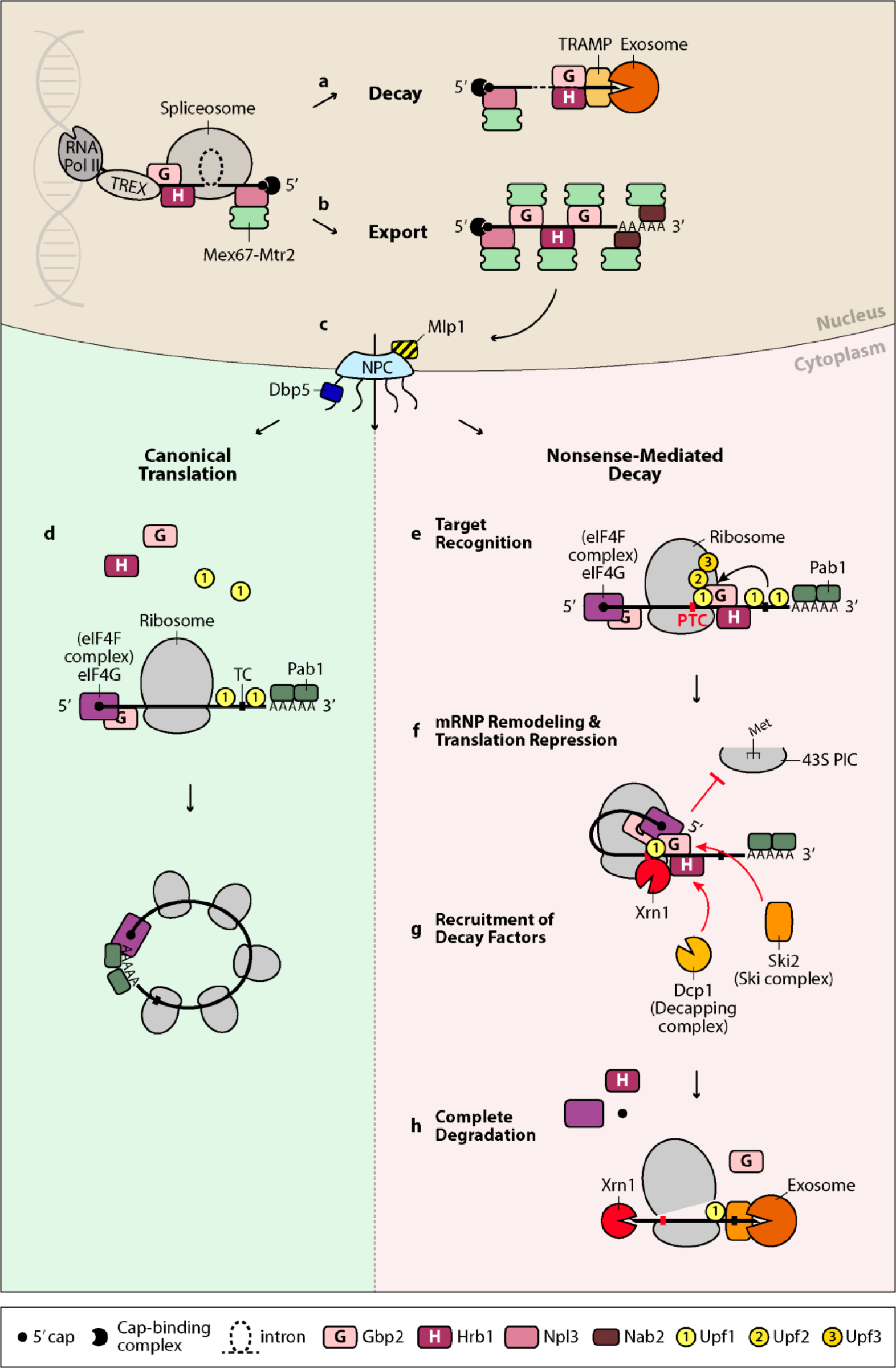
Model for the mRNA quality control functions of Gbp2 and Hrb1. Gbp2 and Hrb1 take part in mRNA quality control both in the nucleus and the cytoplasm. In the nucleus, they are loaded onto the nascent transcript through the TREX complex and associate with the late spliceosome. They recruit the TRAMP complex, which, upon errors in splicing, guides the faulty transcript to the nuclear exosome for degradation (a). In the event of correct splicing, Gbp2 and Hrb1 recruit instead the export factor heterodimer Mex67–Mtr2. Npl3 and Nab2 are two other RNA-binding proteins that associate with the mRNA co-transcriptionally. They also directly recruit Mex67–Mtr2, likely upon correct capping and polyadenylation, respectively, and together this supports proper packaging of the mature messenger ribonucleoprotein (mRNP) (b). The mRNP is quality controlled by Mlp1 and other nuclear pore complex (NPC)-associated proteins before export (c). At the cytoplasmic side of the NPC, the helicase Dbp5 removes Mex67–Mtr2 and Nab2 from the emerging transcript, while Gbp2 and Hrb1 remain bound until translation. Correct transcripts undergo canonical translation, during which Gbp2 and Hrb1 are released (d). Efficient translation is thought to be supported by formation of a closed-loop RNA structure mediated by the interaction between 50-associated eIF4G and poly(A)-binding protein Pab1. On PTC-containing transcripts, Upf1 associates with the terminating ribosome and is activated by the formation of the Upf1– Upf2–Upf3 complex (e). Gbp2 and Hrb1, potentially through dimer- or oligomerization, facilitate mRNP remodeling that brings the 50 end of the transcript into proximity to Upf1, allowing direct contact of Upf1 with eIF4G. Gbp2 and Hrb1 bind to eIF4G and may repress translation initiation of the target transcript (f). Hrb1 promotes recruitment of decapping factor Dcp1, while both proteins promote recruitment of Ski2, a component of the cytoplasmic exosome cofactor Ski complex (g). The decapped RNA is degraded by Xrn1 from the 50 end, while deadenylation and exosome-mediated decay occurs from the 30 end. The helicase activity of Upf1 is required for ribosome disassembly and displacement of bound NMD factors, allowing complete elimination of the transcript (h). RNA Pol II: RNA polymerase II; TREX: transcription/export complex; NPC: nuclear pore complex; TC: termination codon; PTC: premature termination codon; 43S PIC: 43S pre-initiation complex.
Stress response
When cells respond to stress, bulk mRNA is retained in the nucleus to allow the preferential nuclear export of stress responsive transcripts that encode chaperone proteins, important for survival and adaptation to the stress condition. We found that stress responsive transcripts are exported directly by Mex67 without quality control through the guard proteins.
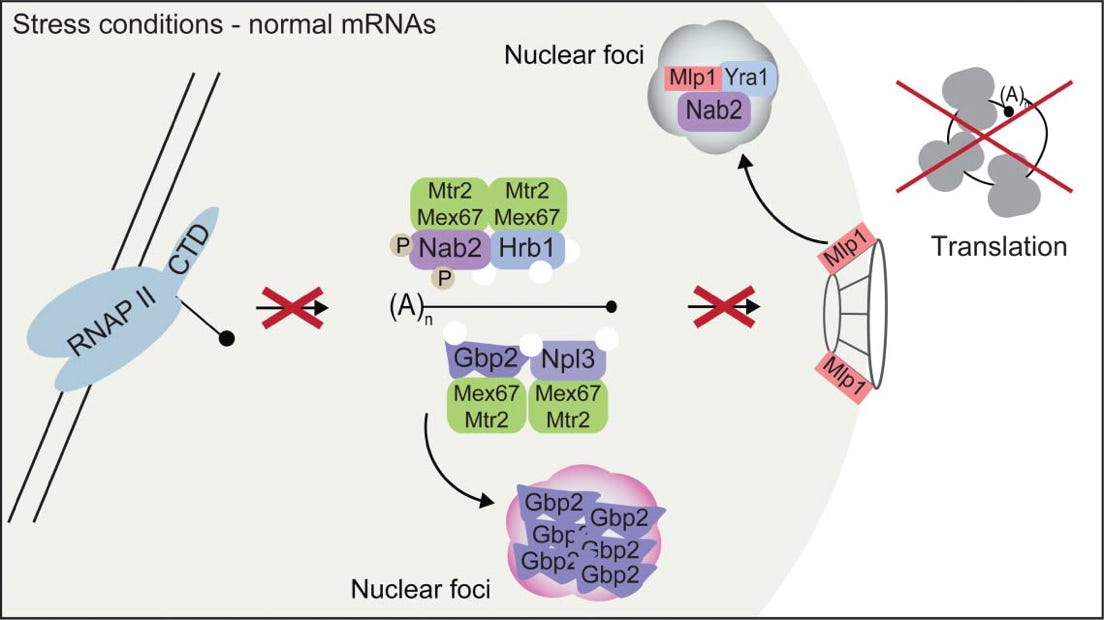
Top: Stress conditions lead to dissociation of Mex67-complexes and a subsequent mRNA export block. Upon stress housekeeping mRNAs are no longer transcribed and Mex67 is dissociated with the guard proteins from mature mRNAs. The recruitment of the guard proteins is prevented by mechanisms, including phosphorylation, aggregation and foci-formation of guard proteins and the NPC gatekeeper Mlp1.
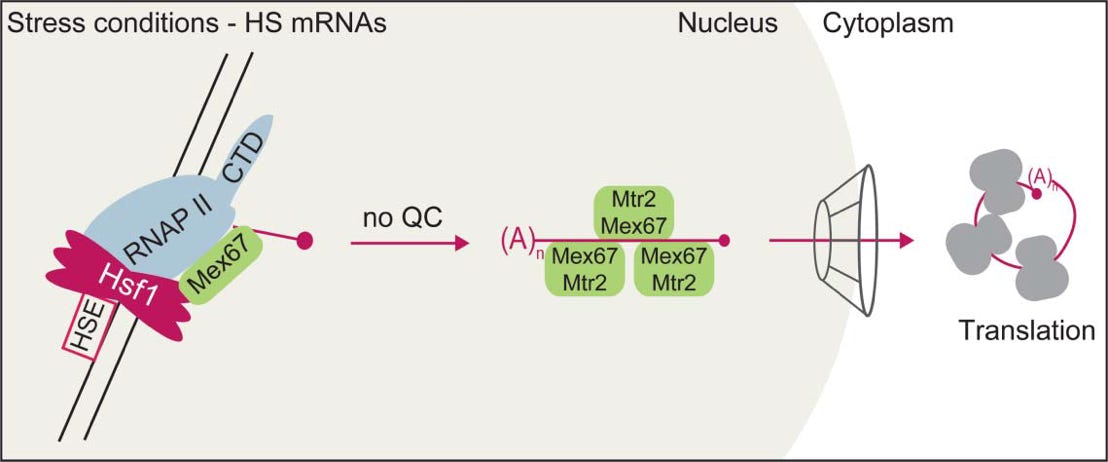
Bottom: Export of HS RNAs. Direct co-transcriptional recruitment of Mex67 to HS mRNAs allows omission of quality control and fast HS mRNA export.
snRNAs
Pre-mRNA splining is mediated by the spliceosome. This molecular machine contains small nuclear (sn)RNAs that are transcribed as longer precursors, which travel into the cytoplasm for maturation. If nuclear export is prevented, these longer snRNAs can be incorporated into the spliceosome, leading to a defective machine and splicing defects.
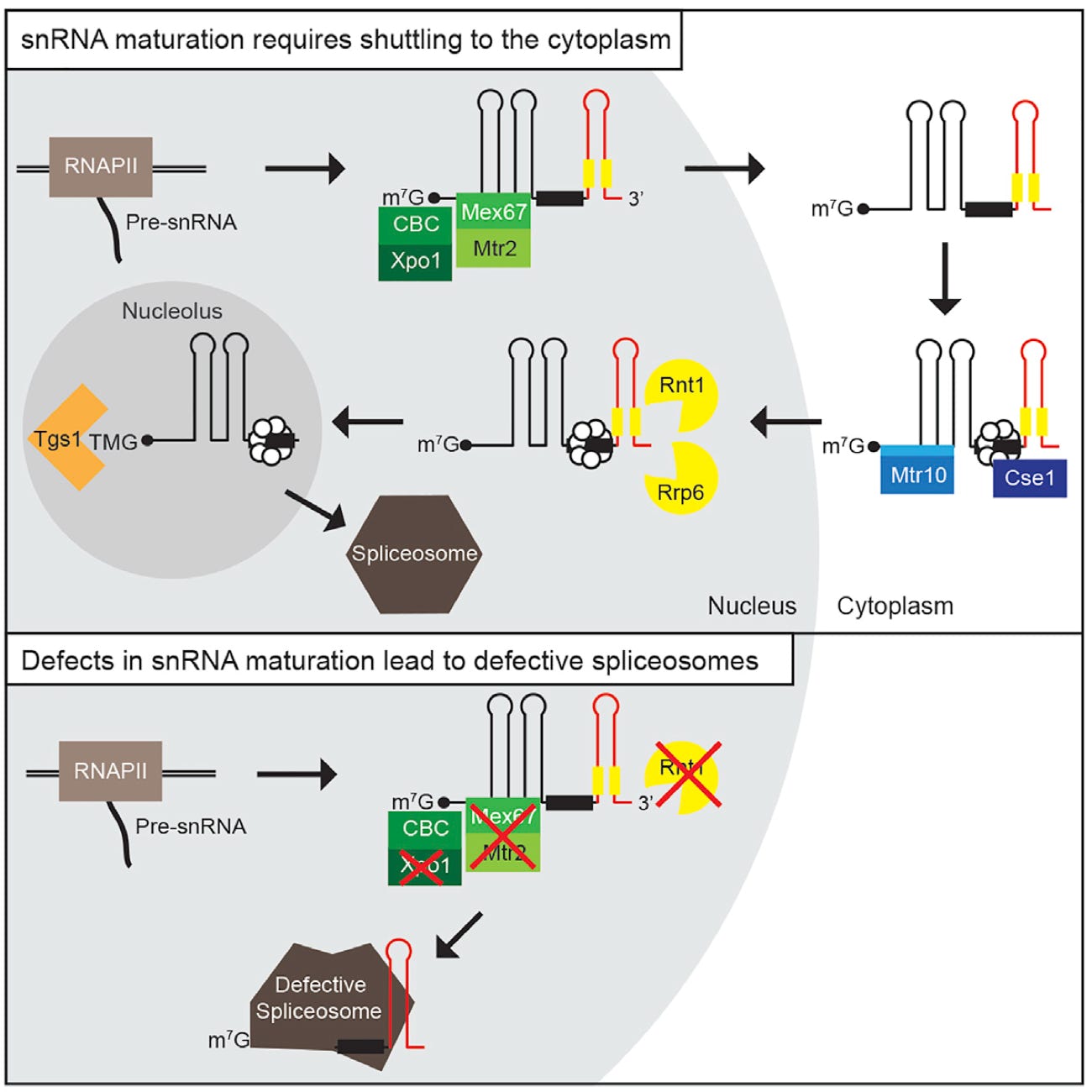
snRNA maturation requires the immediate pre-snRNA nuclear export for quality assurance. Top: Pre-snRNA export to the cytoplasm enables the unhindered assembly of the mature spliceosome.Yeast pre-snRNAs are transcribed in the nucleus and immediately exported into the cytoplasm upon binding of the export receptor Mex67-Mtr2 and the karyopherin Xpo1/Crm1, the latter of which interacts with CBC-boundm7G-caps. Upon export, Mex67 and Xpo1/Crm1 are displaced and the Sm-ring assembles on the pre-snRNP in the cytoplasm. Subsequently, the snRNPs are re-imported back into the nucleus via Mtr10 and Cse1,the latter of which contacts the snRNA via its Sm-ring. In the nucleus, the import receptors dissociate and the pre-snRNA is cleaved by Rnt1 at its 3’-end and further trimmed up to the Sm-ring by the nuclear exosome. Finally, TMG-capping occurs in the nucleolus assisted by SmB, which interactswith Tgs1. This step terminates shuttling, because export receptors cannot be loaded anymore. Mature snRNAs are incorporated into the spliceosome. We have depicted a scheme of the structure of U1 as an example for the snRNAsin this model. Bottom: Pre-snRNAs can be incorporated into the spliceosome, when not exported or processed correctly. The spliceosome cannot distinguish between immature and mature snRNAs. Any reason that retains immature presnRNA in reach of the spliceosome increases the danger of a recruitment of faulty, immature snRNPs, which results in defective spliceosomes and severe splicing defects